|
Part - I
1st January 2005 was a historic deadline for Indian Pharmaceutical Industry (IPI). Before that reverse Engineering was a tool in the hand of Indian Pharmaceutical Industry by which they made it possible any (patented molecule) product by back word Calculation (reverse engineering).
India after over four years of deliberations, daily dallying and protected negotiations finally signed GATT (General agreement on Trade & Tariffs) including Trips (Trade Related Intellectual Property Rights) on 15th April 1994. This is indeed a turning point for Indian Pharmaceutical Industry.
The main impact of TRIPS a pharmaceutical patent is: -
A patent term of 20 years from the date of filing.
Recognition of Product Patents.
Importance of a product to be acceptation on working a Patent.
Compulsory licensing to be confined to special circumference like emergency or abuse of Patent Rights.
Reversal of "Burden of Profit" in infringement action relating to process products.
Before analysis lets look a little back in the industry's last six years performance. The industry is a largely fragmented and highly competitive with a large number of players having interest in it. The following chart shows the breakup of the growth (YoY) of Indian Pharmaceutical Industry in last six years.
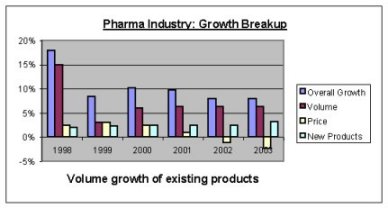
Source: www.pharmatech.com
Next
* Contributed by: -
Dr. R. P. Verma,
Ex. H.O.D. & Dean, Commerce and Business Management Dept.,
Arabinda Bhandari,
Strategic Management Researcher,
Ranchi University, Ranchi.
|